Document control software improves regulatory compliance
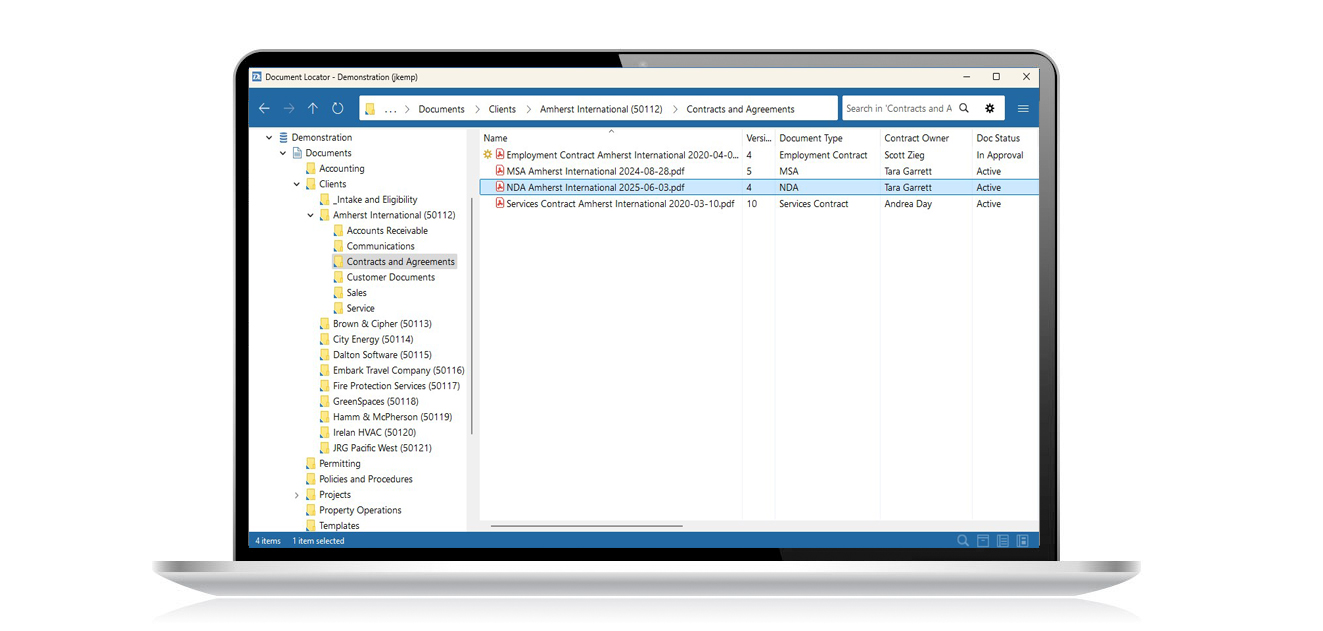
Document control software is essential for managing regulated content where quality and compliance are required. A document control system can help you manage information and files in compliance with regulations and standards like ISO, FDA, Sarbanes-Oxley, and more.
With document control software, you can automate records management policies, control access to information, and secure a complete history of all document activity for auditing and submissions. Wherever information management must comply with regulations and policies, best practices for document control will help avoid risk of penalties and fines.
Improve compliance, reduce risk, and enhance process management with document control software.
Benefits of a document control software
Document control for FDA 21 CFR Part 11
Document control provides effective pharmaceutical document control for the structured management of regulated content across Research and Development, Operations, and Marketing at biotechnology and life sciences companies.
- Easy search and fast document retrieval
- Secure document archiving and audit trail
- Protocols, methods, and test record controls
- Conversion of paper to full-text searchable digital files
- Automation of workflow processes
- Scanning of case and research file archives directly into the repository
- Partner collaboration
Document control solutions for quality management
Document control is essential in the success of quality management. Quality documentation like CAPAs, SOPs, non-conformance reports, employee training, and work instructions are managed within a document control repository that provides for version tracking, approvals, notifications, policy administration, and more.
Advanced features of document control software
Document Control Workflow
Fully-customizable document workflows with notifications, approvals, and logging of activity history.
Reporting
Analyze document and process trends with integrated reporting.
Auditing
Record a history of access, changes, and approvals in the document control system with audit logging.
Document Version Control
Records a history of all changes using document version control, and manages access to version history.
Electronic signatures
Facilitates digital signatures from within the document control system via integrations.
Security
Security settings manage access within the document control system.
Document Imaging
Document scanning digitizes and manages paper files, electronically.
Read more
Learn more
Be ready for the next audit
Employee files under control
Structured project files
Automate business processes
Ready for a Demo?
Take the first step towards streamlining your processes and enhancing collaboration with Document Locator. Request a demo today and discover how our document control solution can help your organization.
Fill out the form to get started.




