A Unified Approach to Nonconformance
Less Manual Effort, Faster Resolutions
Nonconformance isn’t just a quality issue, it’s a business risk. DL QualityCore provides a unified solution to streamline nonconformance processes, reduce manual effort and safeguard compliance. Your team will have the tools to report, investigate and resolve issues efficiently so you can lower the cost of production, shorten resolution times and free your organization to focus on innovation and growth.
How It Works
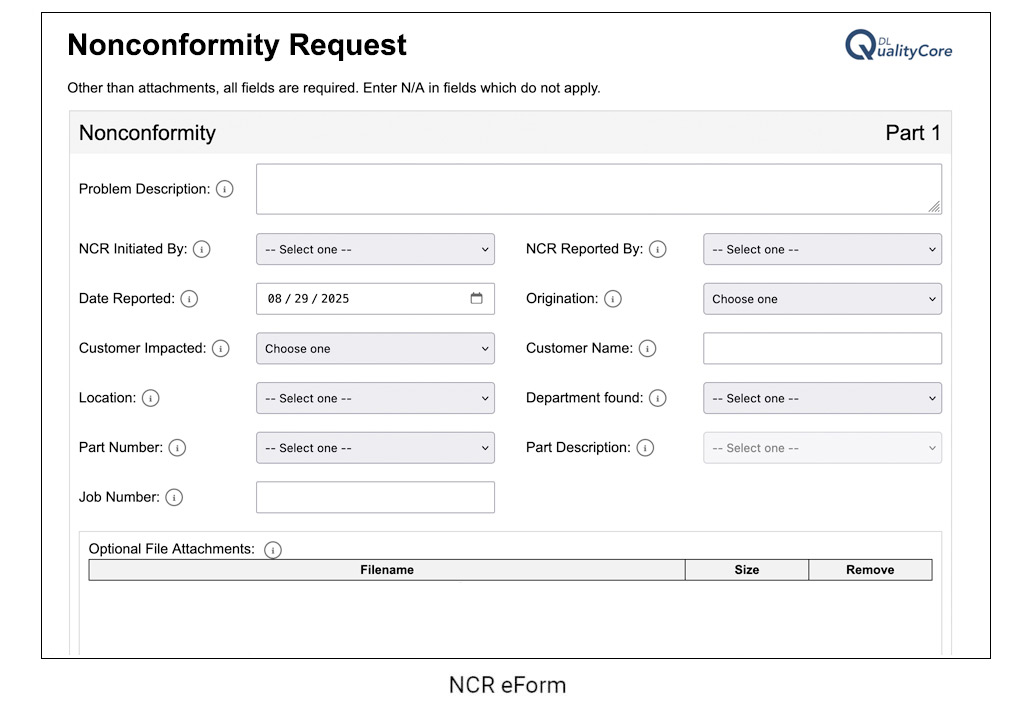
DL QualityCore streamlines nonconformance management by automating the entire process. Teams can quickly create NCRs with eForms, attach supporting files, and route them through workflows that include notifications and escalation rules. Corrective actions are linked directly to each record, ensuring issues are investigated and resolved without delay.
Every completed nonconformance is stored as a permanent PDF record for full traceability. All forms, actions, and attachments are captured automatically, creating a complete audit trail.
Create, assign and track NCRs with customizable workflows, email notifications and escalation rules.
Link corrective actions directly to NCRs. Track progress, assign tasks and verify effectiveness to ensure issues are fully resolved.
Simplify nonconformance reporting with user-friendly electronic forms that can be completed quickly, reducing errors and encouraging adoption across the organization.
Include photos, documents, test results, or any other relevant files within each nonconformance record, ensuring complete context and traceability in one place.
When a nonconformance is completed, all documentation (forms, actions, and attachments) is automatically compiled into a permanent PDF record, ensuring complete traceability and easy access for audits or future reference.
Nonconformance FAQs
A nonconformance is any product, process, or service that fails to meet a requirement, whether from internal procedures, customer expectations, or regulatory standards.
A nonconformance is the issue itself. An NCR (Nonconformance Report) documents the issue. A CAR (Corrective Action Request) is the process to investigate and eliminate root causes.
Poorly handled a nonconformance can lead to recalls, regulatory fines, reputational damage, and higher operational costs.
Nonconformances are logged through a standardized form in a digital system, capturing details such as issue description, date, location, and responsible parties.
The record is reviewed, corrective or preventive actions may be assigned, and once resolved, it is finalized and stored for audit and compliance purposes.
Yes, finalized NCRs are stored permanently as auditable records, supporting regulatory and quality audits as well as continuous improvement.
Trend analysis highlights recurring issues, helping organizations target root causes and prevent repeat problems.
By catching issues early, reducing rework, preventing recalls, and streamlining workflows, organizations cut costs and protect profitability.
Say what you do. Do what you say. Prove it!
Say It. Do It. Prove It! With DL QualityCore
DL QualityCore embodies the core compliance principle of “Say what you do. Do what you say. Prove it.” by ensuring that policies, procedures, and records are documented, followed, and verifiable. With structured document control and automated workflows, organizations can clearly define their processes, ensuring consistency and compliance with industry regulations.
By enforcing version control, training requirements, and automated approvals, DL QualityCore helps teams do what they say—ensuring that employees follow documented procedures. Robust reporting, audit trails, and records management allow organizations to prove it, providing the transparency and traceability needed for audits, inspections, and continuous improvement.