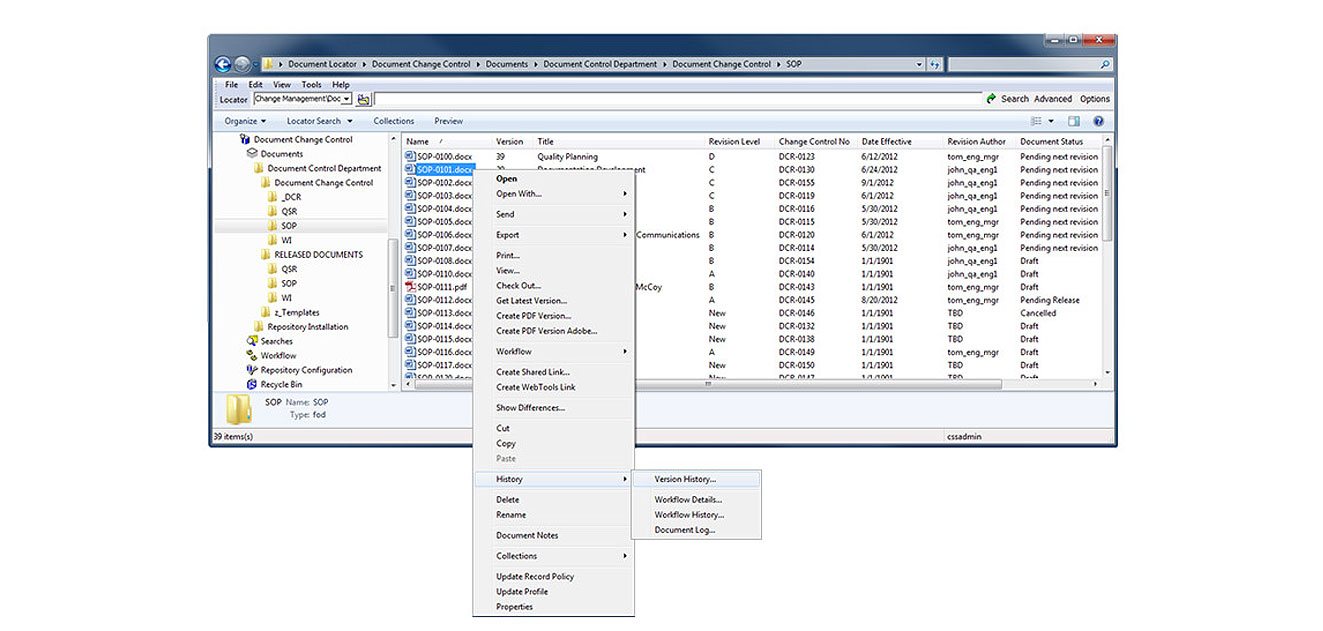
ISO 9001 document control
ISO 9001 document control is essential to a quality management system. Although organizations have flexibility in the way they choose to document their quality management system (QMS), the standard defines how organizations develop the documentation needed in order to demonstrate planning, operation and control of processes, and the implementation and continual improvement of the QMS.
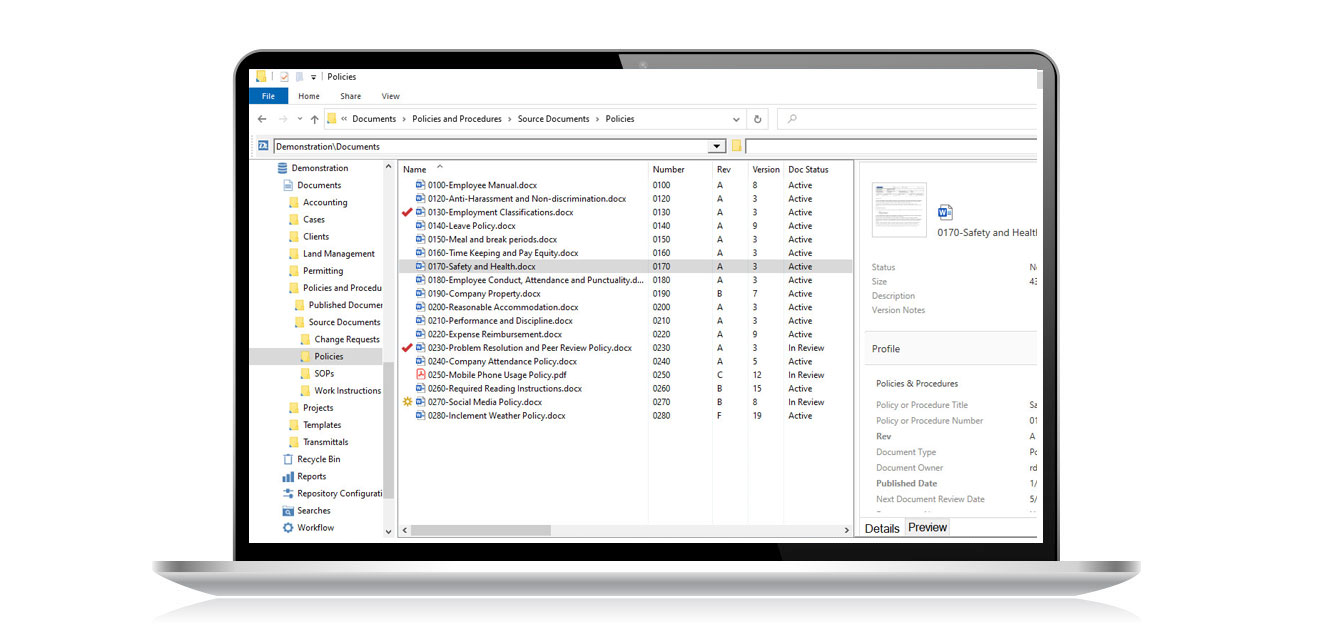
The flexibility and control you need in a Quality Management System for ISO 9001 document control.
Why ISO 9001 document controls are needed
Organizations that want to demonstrate conformity with the requirements of ISO for certification, registration, contractual obligations, or other reasons, need to provide evidence of an effective implementation of a quality management system (QMS). You must be able to provide objective evidence of the effectiveness of processes and the overall quality management system. This objective evidence may be obtained through observation, measurement, test, or other means.
What is a document according ISO 9001?
Documents communicate information, provide evidence of conformity, and allow for knowledge sharing. Documents may come in a number of forms, for example paper, electronic, or images.
ISO 9001 requires an organization establish, document, implement, and maintain a quality management system and continually improve its effectiveness. QMS documentation includes: a documented quality policy and quality objectives; a quality manual, documented procedures; documents needed for effective planning, operation and control of processes; and certain other records required by the international standard. The standard also specifically requires a documented procedure be established, documented, implemented and maintained.
The extent of the QMS documentation may differ from one organization to another due to the size of an organization and its types of activities, and the complexity of their processes.
All the documents that form part of the QMS must be controlled.
General documentation requirements for ISO 9001
Here is an overview of the general documentation requirements of ISO:
Documented quality policy and objectives
ISO defines the requirements of the quality policy and requires that the quality policy is itself a document. It also identifies the requirements for quality objectives.
Quality manual
The quality manual is also a document that must be controlled. The minimum content for a quality manual is specified by ISO, however, the format and structure of the manual can vary based on the needs of the organization. For example, a small organization may include the description of its entire QMS within a single manual, including the documented procedures, while a large organization may require several manuals and a more complex hierarchy of documentation.
Documented procedures
ISO requires documented procedures that must be controlled. These activities include:
- Control of documents
- Control of records
- Internal audits
- Control of nonconforming product
- Corrective actions
- Preventive actions
Documents needed for effective planning, operation and control of processes
Additional documents may be needed to demonstrate an effective QMS. These documents must be controlled as well. Examples of these are:
- Quality policy
- Quality objectives
- Quality manual
- Process maps, process flow charts and/or process descriptions
- Organization charts
- Specifications
- Work and/or test instructions
- Documents containing internal communications
- Production schedules
- Approved supplier lists
- Test and inspection plans
- Quality plans
Additional records
Additional records may also be needed to demonstrate conformity of processes, products, and the quality management system according to ISO. While the requirements for the control of such records are different from other documents, ISO does mandate that they be controlled.
Document control software and ISO 9001
Document Locator is document control software that provides the essential capabilities for controlling documents according ISO regulations. Document control software allows you to automate records management policies, control access to information, and secure a complete history of all document activity for auditing. Examples include:
Approving documents for ISO 9001
Document approval procedures define which files are final and approved and who made the approval. They record acceptance of documents, policies, work instructions, handbooks, and more.
In Document Locator, document approval steps are designed to support business requirements. Documents are routed and notifications are sent informing people of their tasks in review, acceptance and sign-off. As files are electronically routed, all approval actions are recorded in the system. Routing can be configured to automate tasks based on approval status.
Providing access to documents for ISO 9001
Document Locator provides controlled yet flexible access to documents in your quality management program. Security determines who has access to which files, and what actions they can perform. Access to files is possible via the native Windows Explorer integration, online in a Web browser, or on a mobile device.
Read more
Learn more
Be ready for the next audit
Employee files under control
Structured project files
Automate business processes
Ready for a Demo?
Take the first step towards streamlining your processes and enhancing collaboration with Document Locator. Request a demo today and discover how our document control solution can help your organization.
Fill out the form to get started.